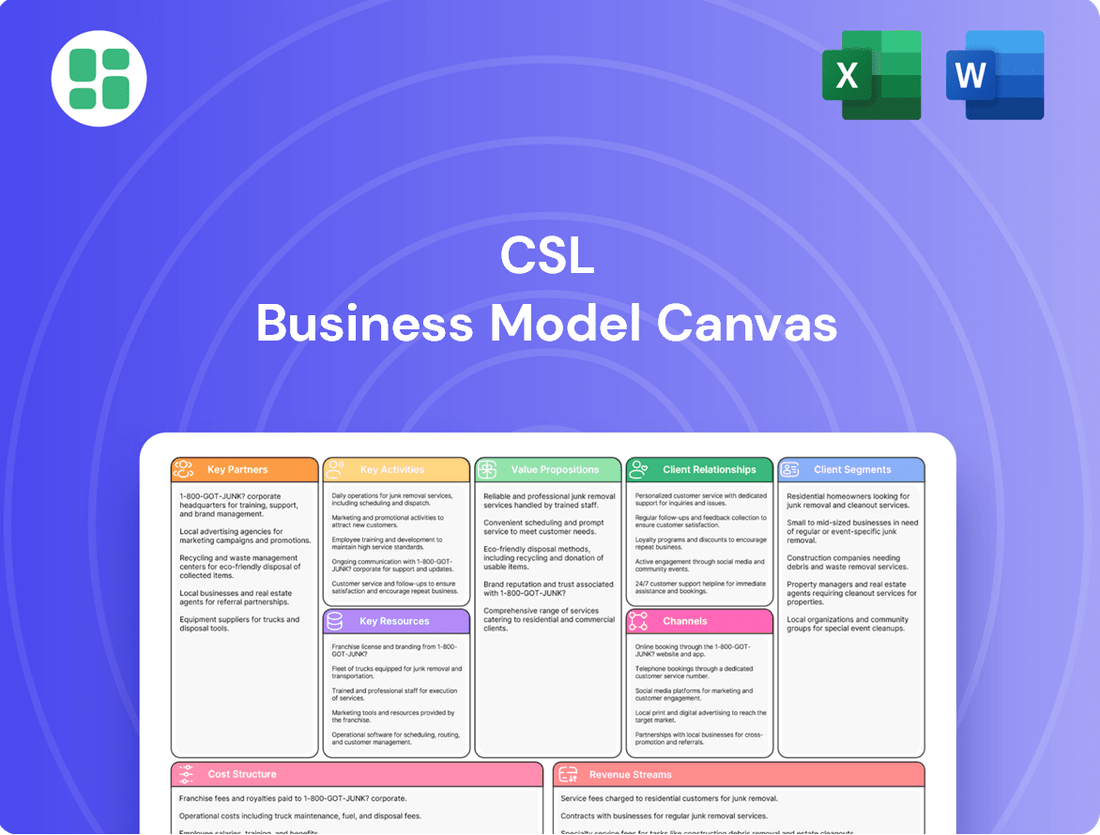
CSL Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
CSL Bundle
Uncover the strategic engine driving CSL's success with our comprehensive Business Model Canvas. This detailed breakdown illuminates their customer segments, value propositions, and revenue streams, offering a clear roadmap to their market dominance. Ready to dissect a winning strategy?
Partnerships
CSL's business model is fundamentally built upon its extensive network of plasma donors and collection centers, operated primarily under the CSL Plasma brand. This partnership is the bedrock for sourcing the essential raw material, plasma, which is then transformed into life-saving therapies by CSL Behring. The company's commitment to expanding and optimizing these centers directly impacts its ability to meet the growing global demand for its products.
In 2023, CSL operated over 300 plasma collection centers worldwide, reflecting a significant investment in this crucial upstream component. The consistent growth in plasma collections, a key performance indicator for CSL Plasma, directly fuels the production pipeline for CSL Behring's therapies. This symbiotic relationship ensures a stable supply chain, vital for maintaining market leadership and patient access to critical treatments.
CSL's innovation engine thrives on collaborations with academic and research institutions, including universities and biotech startups. These partnerships are vital for identifying novel therapeutic targets and advancing groundbreaking technologies like gene therapies and advanced vaccines.
In 2024, CSL continued to leverage these relationships, engaging in licensing deals and strategic option agreements. These collaborations are designed to bolster CSL's scientific expertise and broaden its portfolio of innovative therapies, ensuring a robust pipeline for future growth.
CSL Seqirus actively collaborates with governmental and public health organizations across the globe, positioning itself as a crucial partner in pandemic preparedness and the reliable supply of seasonal influenza vaccines. These vital relationships frequently translate into long-term contracts for vaccine production, strategic stockpiling initiatives, and the capacity for swift action when new health threats emerge.
Healthcare Providers and Hospitals
Healthcare providers, including hospitals and clinics, are critical partners for CSL, facilitating the delivery and administration of its specialized therapies. These collaborations are fundamental to ensuring CSL's innovative treatments reach patients, particularly within intricate healthcare environments. For instance, in 2024, CSL Behring's immunoglobulin therapies were vital for managing primary immune deficiencies, a condition requiring ongoing medical supervision within hospital and clinic settings.
Strong relationships with medical professionals are paramount for accurate patient diagnosis, promoting treatment adherence, and securing broad market access for CSL's product portfolio. In 2023, CSL invested significantly in medical education programs aimed at healthcare providers to enhance understanding and appropriate use of its plasma-derived and recombinant therapies. This focus on provider engagement is key to expanding patient reach and improving therapeutic outcomes.
- Distribution Channels: Hospitals and clinics act as the primary points for dispensing CSL's life-saving treatments.
- Clinical Expertise: Healthcare professionals provide the specialized knowledge required for the safe and effective administration of CSL's therapies.
- Market Access: Partnerships with providers are essential for gaining formulary acceptance and reimbursement within healthcare systems.
- Patient Support: Collaborations enable integrated patient care, from diagnosis through ongoing treatment management.
Strategic Pharmaceutical and Biotech Alliances
CSL actively cultivates strategic pharmaceutical and biotech alliances, engaging in licensing agreements to bolster its product pipeline and expand market access. These collaborations are crucial for accessing novel technologies and therapeutic areas that complement CSL's internal innovation. For instance, in 2024, CSL continued to explore partnerships in areas like self-amplifying mRNA vaccines and innovative treatments for rare diseases, aiming to accelerate product development and commercialization.
These alliances serve to leverage external scientific expertise and share the inherent risks associated with research and development investments. By pooling resources and knowledge, CSL can de-risk its R&D portfolio, allowing it to pursue a broader range of promising candidates. This collaborative approach is a cornerstone of CSL's strategy to maintain a competitive edge in the dynamic biopharmaceutical landscape.
- Product Pipeline Enhancement: Partnerships provide access to complementary technologies and late-stage assets.
- Market Reach Expansion: Alliances can facilitate entry into new therapeutic areas and geographic markets.
- R&D Risk Mitigation: Sharing development costs and expertise reduces the financial burden on CSL.
- Innovation Acceleration: Collaborations speed up the translation of scientific discoveries into viable treatments.
CSL's key partnerships are diverse, ranging from plasma donors to global health organizations and healthcare providers.
These collaborations are essential for sourcing raw materials, advancing research, ensuring vaccine availability, and delivering therapies to patients.
In 2023, CSL operated over 300 plasma collection centers, a testament to its partnership with donors.
CSL Seqirus's partnerships with governments in 2024 were crucial for influenza vaccine supply, with significant government contracts anticipated.
| Partner Type | Role | 2023/2024 Relevance |
|---|---|---|
| Plasma Donors | Source of raw material | Over 300 collection centers operated |
| Academic/Research Institutions | Innovation and technology access | Licensing deals and option agreements in 2024 |
| Govt./Public Health Orgs | Pandemic preparedness, vaccine supply | Long-term contracts for influenza vaccines |
| Healthcare Providers | Therapy delivery and administration | Essential for immunoglobulin therapies in 2024 |
| Pharma/Biotech Alliances | Pipeline enhancement, R&D risk sharing | Focus on mRNA vaccines and rare diseases in 2024 |
What is included in the product
A structured framework detailing CSL's approach to customer relationships, revenue streams, and key resources. It outlines how CSL creates, delivers, and captures value across its operational segments.
Provides a structured framework to systematically identify and address business model weaknesses.
Helps to pinpoint and resolve strategic inconsistencies by visualizing all key business elements.
Activities
CSL's core activity is a robust research and development engine focused on creating groundbreaking biotherapies and vaccines. This commitment is evident in their substantial investments across key therapeutic areas like immunology, haematology, nephrology, and influenza, aiming to address unmet medical needs.
In 2024, CSL continued to strategically streamline its R&D operations, enhancing efficiency. The company is increasingly leveraging external collaborations and partnerships to accelerate innovation and expand its pipeline, recognizing the value of diverse scientific expertise.
CSL Behring's core operations revolve around collecting human plasma via its extensive global network of CSL Plasma centers. This vital raw material is the foundation for all its life-saving therapies.
Once collected, the plasma is subjected to sophisticated fractionation and purification processes. These complex steps are crucial for isolating and concentrating the therapeutic proteins needed for CSL's products.
These activities directly enable the production of essential immunoglobulins, albumin, and other critical plasma-derived medicines. In 2023, CSL's plasma collection volume contributed significantly to its revenue, underscoring the importance of this key activity.
Biopharmaceutical manufacturing is CSL's core operational activity, encompassing the production of life-saving therapies derived from plasma, recombinant proteins, and influenza vaccines across its global network of advanced facilities. This intricate process demands rigorous adherence to strict regulatory standards to guarantee product safety, efficacy, and consistent supply.
CSL's commitment to manufacturing excellence is evident in its ongoing strategic investments. For instance, the company has been expanding its plasma processing capabilities and integrating advanced automation technologies to boost efficiency and capacity, ensuring it can meet growing global demand for its critical medicines.
Clinical Trials and Regulatory Affairs
CSL's core operations revolve around meticulously conducting clinical trials, spanning Phase I through Phase IV, to rigorously assess the safety and effectiveness of its innovative therapies. This extensive testing is fundamental to building a strong scientific foundation for each product.
Navigating the intricate and ever-evolving global regulatory landscape is another critical activity. CSL's expertise in this area ensures that its products meet the stringent requirements of health authorities worldwide, facilitating market entry and continued availability.
In 2024, CSL continued its commitment to advancing its pipeline, with significant investments in clinical development programs. For instance, its influenza vaccine franchise, a major revenue driver, relies on ongoing trials to demonstrate efficacy against circulating strains, a process that involves extensive data collection and analysis across diverse patient populations.
- Clinical Trial Execution: Managing multi-phase trials to gather robust safety and efficacy data for new and existing products.
- Regulatory Submission and Approval: Preparing and submitting comprehensive dossiers to global health authorities for product licensing and ongoing compliance.
- Post-Market Surveillance: Conducting ongoing studies and monitoring to ensure continued product safety and effectiveness after market launch.
- Global Regulatory Expertise: Maintaining a deep understanding of and adherence to diverse regulatory requirements in over 100 countries where CSL products are available.
Global Sales, Marketing, and Distribution
CSL actively manages the global sales, marketing, and distribution of its biotherapies and vaccines. This critical function ensures their products reach healthcare providers, governments, and patients worldwide. A key aspect involves crafting effective market access strategies and fostering strong relationships with medical professionals.
The company's distribution network is designed for efficiency, ensuring timely delivery of essential medicines. CSL is focused on expanding its product portfolio and increasing its geographic reach to serve more markets. For instance, in fiscal year 2023, CSL reported strong growth, with revenue reaching approximately $13.3 billion, reflecting the success of its global commercialization efforts.
- Global Reach: CSL's products are available in over 100 countries.
- Market Access: Strategies are tailored to navigate diverse regulatory and reimbursement landscapes.
- Supply Chain Excellence: Robust logistics ensure product integrity and availability.
- Portfolio Expansion: Continuous efforts to introduce new therapies and vaccines globally.
CSL's key activities encompass a comprehensive approach to biopharmaceutical development and commercialization. This includes a relentless focus on research and development to create innovative therapies, the critical process of collecting and fractionating human plasma, and the meticulous manufacturing of life-saving medicines. Furthermore, CSL executes rigorous clinical trials, navigates complex global regulatory environments, and manages the worldwide sales and distribution of its products.
These activities are supported by significant financial commitments and operational achievements. For example, CSL's revenue for fiscal year 2023 reached approximately $13.3 billion, demonstrating the success of its global commercialization efforts.
| Key Activity Area | Description | 2023 Financial Impact (Approx.) | 2024 Focus Areas |
|---|---|---|---|
| Research & Development | Creating novel biotherapies and vaccines for unmet medical needs. | Significant investment in pipeline expansion. | Streamlining R&D, leveraging external collaborations. |
| Plasma Collection & Fractionation | Operating a global network of plasma centers for raw material collection. | Crucial for revenue generation. | Expanding plasma processing capabilities. |
| Biopharmaceutical Manufacturing | Producing plasma-derived therapies, recombinant proteins, and vaccines. | Ensuring consistent supply of critical medicines. | Integrating advanced automation technologies. |
| Clinical Trials & Regulatory Affairs | Executing multi-phase trials and navigating global regulatory landscapes. | Foundation for product approval and market access. | Advancing influenza vaccine programs, ensuring compliance. |
| Sales, Marketing & Distribution | Reaching healthcare providers and patients globally. | Revenue of $13.3 billion in FY23. | Expanding geographic reach and product portfolio. |
Delivered as Displayed
Business Model Canvas
The CSL Business Model Canvas you are previewing is the actual document you will receive upon purchase. This means you're seeing the exact structure, content, and formatting that will be delivered, ensuring no surprises. Once your order is complete, you'll gain full access to this comprehensive and ready-to-use business planning tool.
Resources
CSL's intellectual property, particularly its patents covering plasma-derived therapies and recombinant products, forms a cornerstone of its business model. This robust patent portfolio safeguards its innovative treatments and manufacturing techniques, providing a significant competitive edge. For instance, CSL's ongoing commitment to research and development, which saw significant investment in 2024, directly fuels the growth and protection of this vital intellectual capital.
CSL's global plasma collection network is a cornerstone of its business, acting as the primary source for the plasma needed to create its life-saving therapies. This network is not just extensive but is continually being enhanced with technology, such as the Rika automated plasma collection system, which improves donor experience and collection efficiency. As of the first half of fiscal year 2024, CSL reported a significant increase in plasma collections, underscoring the network's growing capacity and importance.
CSL's advanced manufacturing facilities are the backbone of its biopharmaceutical production, encompassing state-of-the-art fractionation plants and vaccine manufacturing sites. These world-class assets are essential for CSL's ability to deliver high-quality, high-volume biotherapies and vaccines globally.
The company's commitment to cutting-edge production is underscored by recent accolades, such as those received by its Broadmeadows plant, recognizing its excellence in biopharmaceutical manufacturing. This focus on advanced technology ensures CSL can meet the growing demand for its life-saving products.
Highly Skilled Scientific and Medical Talent
CSL's foundation rests on a highly skilled scientific and medical talent pool, numbering over 32,000 employees globally. This diverse workforce includes top-tier scientists, dedicated researchers, experienced medical professionals, and manufacturing specialists. Their collective expertise is the engine driving CSL's innovation, ensuring operational efficiency, and maintaining a strong focus on patient well-being.
The company's commitment to talent development is evident in its continuous investment in training and fostering a culture that champions scientific excellence. This strategic approach is crucial for CSL's ongoing expansion and success in the biopharmaceutical industry.
- Global Workforce: Over 32,000 employees worldwide.
- Expertise Spectrum: Scientists, researchers, medical professionals, manufacturing experts.
- Core Drivers: Innovation, operational excellence, patient-centricity.
- Strategic Investment: Talent development and a culture of scientific excellence.
Regulatory Approvals and Brand Reputation
CSL's extensive portfolio of regulatory approvals, including those from the FDA, EMA, and other global health authorities, represents a critical intangible asset. These approvals are essential for bringing its life-saving therapies and vaccines to market, with CSL Behring's immunoglobulin products and influenza vaccines being prime examples. As of fiscal year 2023, CSL continued to invest heavily in research and development, securing new approvals and expanding indications for existing treatments, underscoring the ongoing value of this resource.
Complementing its regulatory successes, CSL cultivates a robust brand reputation built on decades of commitment to quality, safety, and scientific innovation. This strong standing within the medical community and among patient advocacy groups fosters trust and facilitates market access. For instance, CSL's influenza vaccine, Fluad Quadrivalent, has consistently demonstrated strong performance and physician preference, a testament to its established reputation.
- Regulatory Approvals: CSL holds numerous product approvals across key global markets, enabling commercialization of its diverse therapeutic offerings.
- Brand Reputation: A strong reputation for quality, safety, and scientific integrity builds trust with healthcare professionals and patients.
- Market Access: Regulatory approvals and brand trust directly translate into enhanced market access and commercial viability for CSL's products.
CSL's key resources are its intellectual property, global plasma collection network, advanced manufacturing facilities, skilled workforce, and regulatory approvals/brand reputation. These elements collectively enable the company to develop, produce, and distribute life-saving therapies and vaccines worldwide. The company's significant investments in R&D and infrastructure in 2024 and beyond highlight the ongoing importance of these resources.
| Key Resource | Description | Supporting Data/Fact |
| Intellectual Property | Patents covering plasma-derived and recombinant therapies. | Significant R&D investment in 2024 fuels patent growth. |
| Plasma Collection Network | Extensive global network for plasma sourcing. | Increased plasma collections reported in H1 FY2024. |
| Manufacturing Facilities | State-of-the-art fractionation and vaccine production sites. | Broadmeadows plant received excellence accolades. |
| Human Capital | Over 32,000 employees with diverse scientific and medical expertise. | Focus on talent development and scientific excellence. |
| Regulatory & Brand | Global product approvals and strong reputation for quality. | Fluad Quadrivalent demonstrates physician preference and market trust. |
Value Propositions
CSL delivers essential, often life-saving biotherapies for individuals facing rare and severe diseases like immune deficiencies, hemophilia, and hereditary angioedema. These treatments dramatically enhance patients' lives and health outcomes, especially for those with few alternatives.
The company's dedication to tackling critical, unmet medical needs underscores its core mission. In 2024, CSL's Plasma-Derived Therapies segment, a key area for these treatments, continued to be a significant revenue driver, demonstrating the substantial market demand for these specialized medicines.
CSL Seqirus provides a broad range of influenza vaccines, essential for safeguarding public health and bolstering pandemic readiness. Their cutting-edge vaccine technologies and swift response mechanisms are vital in shielding populations globally from infectious disease threats.
The company is actively developing advanced vaccine platforms, such as self-amplifying mRNA technology, to enhance future preparedness. In 2023, Seqirus reported strong sales growth, driven by demand for its seasonal influenza vaccines and its expanding pipeline.
CSL Vifor's value proposition centers on enhancing the quality of life for individuals managing chronic conditions, particularly iron deficiency and nephrology. Their therapeutic solutions directly address significant unmet needs, offering patients better ways to manage their diseases and potentially avoid severe complications.
For instance, in 2023, CSL reported that its iron deficiency and nephrology portfolio, significantly bolstered by the Vifor acquisition, contributed to substantial revenue growth, underscoring the market demand for these life-improving treatments. This focus allows patients to lead more fulfilling lives despite their chronic illnesses.
Scientific Innovation and R&D Excellence
CSL's dedication to scientific innovation and R&D excellence is a cornerstone of its value proposition, fueling the creation of groundbreaking therapies. This commitment is evident in their diverse portfolio, which spans plasma-derived medicines to cutting-edge gene therapies. In fiscal year 2023, CSL reported R&D expenses of approximately $1.5 billion, underscoring their significant investment in future medical advancements.
Their robust R&D pipeline, bolstered by strategic investments in emerging technologies, firmly places CSL at the vanguard of biotechnology. This forward-thinking approach ensures a consistent flow of novel and enhanced treatments for patients worldwide. CSL’s focus on innovation is designed to address unmet medical needs and drive long-term growth.
- Plasma-Derived Therapies: CSL Behring is a global leader in plasma therapies, treating rare and serious diseases.
- Gene Therapies: CSL is actively developing gene therapies, including for hemophilia, representing a significant area of future growth.
- Biotechnology Advancements: Strategic partnerships and acquisitions, such as the acquisition of Vita-Medica in 2023, further bolster their R&D capabilities.
- R&D Investment: CSL’s consistent investment in R&D, exceeding $1.5 billion in FY23, highlights their commitment to scientific discovery.
Reliable and Global Supply of Essential Biologics
CSL's value proposition hinges on providing a dependable global supply of vital biologics. Through its extensive manufacturing and distribution network, CSL reaches patients in over 100 countries, ensuring uninterrupted access to critical therapies and vaccines. This operational strength is paramount for treatments requiring consistent patient use.
The company's commitment to reliability is underscored by ongoing investments in expanding its production capacity. For instance, CSL's significant capital expenditures, such as the approximately $750 million investment in its new gene therapy manufacturing facility in Australia, directly bolster its ability to meet growing global demand for complex biologics.
- Global Reach: Serves patients in over 100 countries with essential biotherapies.
- Operational Efficiency: Maintains a reliable and consistent supply chain.
- Capacity Expansion: Significant investments, like the $750M Australian facility, enhance production capabilities to meet demand.
CSL offers life-changing therapies for rare and serious diseases, significantly improving patient lives where few alternatives exist. Their focus on unmet medical needs, particularly in plasma-derived therapies, demonstrates a strong market demand. The company’s commitment to innovation is evident in their substantial R&D investments, exceeding $1.5 billion in fiscal year 2023, ensuring a pipeline of advanced treatments.
CSL provides essential influenza vaccines, bolstering global public health and pandemic preparedness through advanced technologies. Their strategic investments, such as the $750 million facility in Australia, enhance production capacity to ensure reliable global supply of critical biologics to over 100 countries.
| Value Proposition | Description | Key Data/Fact |
|---|---|---|
| Life-Saving Therapies | Treats rare and serious diseases, improving patient health outcomes. | Plasma-Derived Therapies segment is a significant revenue driver. |
| Public Health Protection | Offers essential influenza vaccines and pandemic preparedness. | Seqirus reported strong sales growth in 2023. |
| Chronic Condition Management | Enhances quality of life for patients with iron deficiency and nephrology. | Iron deficiency and nephrology portfolio contributed to substantial revenue growth in 2023. |
| Scientific Innovation | Develops groundbreaking therapies through significant R&D investment. | FY23 R&D expenses were approximately $1.5 billion. |
| Global Supply Reliability | Ensures uninterrupted access to critical therapies worldwide. | Serves patients in over 100 countries; investing $750M in Australian facility. |
Customer Relationships
CSL actively cultivates robust relationships with patient advocacy organizations, recognizing their vital role in empowering individuals with rare and serious diseases. These partnerships are crucial for understanding patient needs and developing effective support systems.
The company offers a range of patient support programs designed to ease the burden of managing complex conditions. These programs encompass vital educational resources, critical financial assistance initiatives, and guidance to connect patients with essential healthcare services. For instance, CSL Behring’s patient assistance programs provided over $1.2 billion in financial support to eligible patients in fiscal year 2023, highlighting a significant commitment to accessibility.
These comprehensive support mechanisms underscore CSL's deeply ingrained patient-centric philosophy, extending far beyond the mere provision of life-saving therapies. By investing in patient well-being and access, CSL solidifies its role as a trusted partner in the healthcare journey.
CSL cultivates robust partnerships with healthcare professionals, including physicians, specialists, nurses, and pharmacists. This engagement is achieved through comprehensive medical education programs and scientific symposia, ensuring providers are up-to-date on CSL’s innovative therapies and their optimal application. For instance, CSL Behring's medical affairs teams actively participate in over 100 scientific events annually, directly engaging thousands of healthcare professionals.
These direct interactions are crucial for disseminating the latest clinical evidence and fostering a deep understanding of CSL's product portfolio. By prioritizing trust and providing ongoing clinical support, CSL empowers healthcare providers to make informed decisions, ultimately benefiting patient care. In 2024, CSL invested over $500 million in research and development, a significant portion of which directly supports the clinical education and data sharing vital to these professional relationships.
CSL actively engages with leading researchers and key opinion leaders (KOLs) in critical therapeutic areas. These collaborations are foundational for advancing scientific understanding and informing CSL's clinical development strategies. For instance, CSL's ongoing partnerships in rare diseases have directly contributed to shaping treatment paradigms, as evidenced by the increased adoption of novel therapies informed by KOL insights.
This deep scientific exchange not only enhances CSL's understanding of disease progression and unmet needs but also solidifies its reputation as a thought leader. In 2024, CSL highlighted several successful research collaborations at major scientific conferences, showcasing how these partnerships influence the development of next-generation therapies and contribute to improved patient outcomes.
Government and Public Health Engagement
CSL cultivates enduring, strategic alliances with governments and public health bodies worldwide. This is particularly evident through CSL Seqirus, a key player in vaccine supply and pandemic preparedness initiatives. In 2024, CSL Seqirus continued its role as a critical supplier of influenza vaccines to numerous national health programs, underscoring its commitment to public health security.
These engagements go beyond simple transactions, encompassing direct negotiations for essential health products, active participation in policy advocacy to shape public health strategies, and collaborative planning for critical health events. The company actively seeks to be recognized as a reliable and indispensable partner in national health frameworks.
- Strategic Partnerships: CSL Seqirus secured multi-year agreements in 2024 with several European countries for the supply of seasonal influenza vaccines, reinforcing its position as a trusted government partner.
- Pandemic Preparedness: The company's ongoing investment in manufacturing capacity and research for pandemic influenza strains aligns with global health security goals, providing essential preparedness capabilities.
- Policy Influence: CSL actively engages with policymakers to advocate for robust public health infrastructure and equitable access to critical medicines and vaccines.
Dedicated Sales and Medical Affairs Teams
CSL leverages dedicated sales and medical affairs teams worldwide to directly connect with healthcare providers, including hospitals, clinics, and individual physicians. These specialized groups are instrumental in disseminating critical product information, responding to medical queries, and offering continuous post-sale support.
These teams are the frontline of CSL's customer engagement, fostering trust and ensuring that healthcare professionals have the knowledge and resources needed for optimal product use. Their efforts are vital for building and sustaining robust professional relationships, directly impacting product adoption and patient outcomes.
- Global Reach: CSL's sales and medical affairs teams operate in numerous countries, tailoring their approach to diverse healthcare landscapes.
- Expertise: Teams are comprised of individuals with deep scientific and clinical knowledge relevant to CSL's product portfolio.
- Relationship Building: A primary focus is on cultivating long-term partnerships with key opinion leaders and healthcare institutions.
- Support and Education: They provide essential product training and ongoing medical education to prescribers and pharmacists.
CSL's customer relationships are multifaceted, built on deep engagement with patients, healthcare professionals, researchers, and governments. The company prioritizes patient support, offering substantial financial aid and educational resources, exemplified by over $1.2 billion in financial support provided by CSL Behring's patient assistance programs in fiscal year 2023.
Professional relationships are fostered through extensive medical education and scientific exchange, with CSL Behring's medical affairs teams engaging thousands of healthcare professionals annually across more than 100 scientific events.
Strategic alliances with governments, particularly via CSL Seqirus, are critical for vaccine supply, with multi-year agreements for seasonal influenza vaccines secured with European nations in 2024.
CSL's dedicated sales and medical affairs teams globally ensure continuous support and education for healthcare providers, building long-term partnerships and driving optimal product use.
Channels
CSL's specialized therapies, particularly its plasma-derived and recombinant products, primarily reach patients through hospitals, clinics, and dedicated infusion centers. These healthcare settings are essential as they possess the necessary infrastructure and trained personnel for the complex administration and ongoing monitoring that CSL's treatments often require.
In 2024, CSL continued to emphasize its partnerships with these healthcare providers to ensure widespread availability of its life-saving therapies. For instance, CSL Behring's focus on rare and serious diseases means that access to specialized treatment centers remains a cornerstone of its patient care model. The company's success hinges on the robust relationships it maintains with these institutions, facilitating direct patient treatment.
CSL leverages a robust network of specialty distributors and wholesalers to navigate the complex cold chain logistics essential for its biopharmaceutical products. These partners are critical for ensuring products reach healthcare providers like hospitals and pharmacies efficiently and compliantly, even in remote areas.
In 2024, the global pharmaceutical cold chain market was valued at approximately $18.1 billion, highlighting the significant infrastructure and expertise required. CSL's reliance on these specialized partners underscores their vital role in maintaining product integrity and accessibility.
Pharmacies, both retail and specialty, are crucial direct channels for CSL to reach patients with specific products like vaccines and certain self-administered therapies. CSL Seqirus actively collaborates with pharmacies to guarantee the efficient and timely distribution of influenza vaccines, a vital component of public health initiatives.
This pharmacy channel's significance is growing, particularly in expanding public access to immunizations and managing the supply chain for specialized medications. In 2024, the US retail pharmacy sector continued to be a major player in vaccine administration, with major chains reporting millions of doses administered annually, underscoring their role in public health.
Government Procurement Agencies
Government procurement agencies are vital channels for CSL, particularly for influenza vaccines and pandemic preparedness. These entities, such as national health services, often make substantial bulk purchases to support public health programs and maintain strategic reserves. CSL Seqirus leverages these relationships to secure large-volume contracts, ensuring widespread vaccine availability and contributing significantly to national health security initiatives.
For instance, in 2024, governments worldwide continued to prioritize vaccine procurement to combat ongoing public health threats. CSL Seqirus's role in supplying these essential medical products highlights the critical nature of these government partnerships. These large-scale contracts not only bolster public health infrastructure but also represent a substantial revenue stream for the company, underscoring their strategic importance.
- Direct Sales Channel: Governments and public health bodies are direct purchasers of vaccines and other critical medical supplies.
- Pandemic Preparedness: These agencies procure stockpiles for national pandemic response, creating significant demand.
- Contractual Agreements: CSL Seqirus secures substantial contracts for national health programs, ensuring large-volume sales.
- Public Health Impact: These partnerships are crucial for public health security and CSL's market presence.
Direct Sales Force
CSL's direct sales force is a cornerstone of its customer relationships, engaging directly with healthcare professionals and institutional buyers. This approach fosters deep understanding and allows for personalized product discussions, crucial for complex biologics.
This direct engagement is vital for market penetration and adoption, particularly for innovative therapies where education and support are paramount. CSL's sales teams are trained to communicate the scientific and clinical value proposition effectively.
- Global Reach: CSL maintains a dedicated global sales force, enabling direct interaction with key stakeholders across diverse markets.
- Relationship Building: The direct sales model prioritizes building strong relationships with healthcare providers and institutional decision-makers.
- Product Education: Sales representatives provide in-depth product education and support, facilitating informed prescribing and purchasing decisions.
- Market Penetration: This channel is instrumental in driving CSL's market share and ensuring successful product launches and sustained growth.
CSL utilizes a multi-channel strategy to deliver its specialized therapies. Key channels include hospitals, clinics, and infusion centers, which are vital for administering complex treatments. Specialty distributors and wholesalers ensure the integrity of cold chain logistics, reaching healthcare providers efficiently.
Pharmacies, both retail and specialty, serve as direct access points for vaccines and self-administered medications, with CSL Seqirus actively participating in public health initiatives. Government procurement agencies are critical for large-volume vaccine contracts and pandemic preparedness, representing a significant revenue stream.
CSL's direct sales force engages with healthcare professionals and institutional buyers, fostering relationships and providing crucial product education for its innovative therapies. This direct approach is instrumental in market penetration and sustained growth.
| Channel | Primary Function | 2024 Relevance/Data Point |
|---|---|---|
| Hospitals, Clinics, Infusion Centers | Administering complex therapies, patient monitoring | Essential infrastructure for rare disease treatments |
| Specialty Distributors & Wholesalers | Cold chain logistics, efficient product delivery | Global cold chain market valued at ~$18.1 billion in 2024 |
| Pharmacies (Retail & Specialty) | Vaccine distribution, self-administered therapies | Major role in US vaccine administration, millions of doses annually |
| Government Procurement Agencies | Bulk vaccine purchases, pandemic preparedness | Key for national health security and large-volume contracts |
| Direct Sales Force | Customer relationships, product education, market penetration | Drives adoption of innovative therapies through direct engagement |
Customer Segments
CSL's customer segment of patients with rare and serious diseases encompasses individuals battling chronic and life-threatening conditions. These include primary immune deficiencies, hereditary angioedema, hemophilia, and alpha-1 antitrypsin deficiency.
These patients depend on CSL's specialized biotherapies for their ongoing medical management. The treatments are often lifelong necessities, crucial for managing their specific conditions and enhancing their overall quality of life.
In 2024, CSL Behring, a key division, continued to be a significant player in these niche markets, with its immunoglobulin therapies, like Privigen, showing strong performance. The company's commitment to these patient groups underscores the critical role its therapies play in addressing unmet medical needs.
Healthcare providers, including physicians, specialists like immunologists and hematologists, and hospitals, are key users of CSL's therapies. Their prescribing habits and adoption of CSL's products directly influence market penetration. For instance, CSL Behring's focus on rare and life-threatening diseases means these providers are essential for patient access to treatments like those for hemophilia and primary immune deficiencies.
In 2024, the global pharmaceutical market, of which CSL is a significant player, continued its upward trajectory, driven by advancements in biologics and specialty medicines. The demand for innovative treatments in areas like immunology and hematology, where CSL has a strong presence, remains robust. This segment of healthcare professionals is crucial for CSL's revenue generation as they are the gatekeepers for patient treatment decisions.
Governments and public health organizations worldwide are key customers for CSL, especially for Seqirus's influenza vaccines and pandemic preparedness tools. These bodies purchase vaccines to administer through national immunization programs and to build up reserves for public health emergencies.
Their primary concerns revolve around safeguarding the health of their populations, preventing the spread of diseases, and ensuring national health security. For instance, in 2024, many governments continued to invest heavily in vaccine procurement and public health infrastructure to combat ongoing respiratory illnesses.
Patients with Iron Deficiency and Kidney Disease
CSL Vifor's core customer segment comprises patients experiencing iron deficiency, particularly those with chronic kidney disease (CKD). This group includes individuals undergoing dialysis, a critical stage where iron management is paramount for treatment efficacy and patient well-being. In 2024, it's estimated that over 37 million adults in the United States have CKD, with a significant portion also suffering from iron deficiency, highlighting the vast unmet need.
Beyond CKD and dialysis patients, CSL Vifor also serves individuals with iron deficiency stemming from other chronic conditions. Heart failure patients, for example, frequently experience iron deficiency, which can exacerbate their symptoms and negatively impact their quality of life. CSL Vifor's therapies aim to address this broader spectrum of iron deficiency, recognizing the interconnectedness of various health challenges.
- Target Population: Patients with iron deficiency, especially those with Chronic Kidney Disease (CKD) and those on dialysis.
- Broader Reach: Includes patients with iron deficiency linked to other chronic conditions like heart failure.
- Market Size Indicator: Over 37 million adults in the US diagnosed with CKD in 2024, many of whom also have iron deficiency.
- CSL Vifor's Role: Providing comprehensive therapeutic solutions to manage the full range of iron deficiency needs in these vulnerable patient groups.
Pharmaceutical Distributors and Wholesalers
Pharmaceutical distributors and wholesalers are vital intermediaries for CSL, purchasing products in large quantities to supply hospitals, clinics, and pharmacies. These partners are critical for ensuring CSL's medicines reach patients efficiently across diverse geographic regions.
Their extensive logistics networks and established relationships within the healthcare system are fundamental to CSL's market penetration and product availability. For instance, in 2024, the global pharmaceutical wholesale market was valued at approximately $1.5 trillion, highlighting the significant scale and importance of these customer segments.
- Intermediary Role: They bridge the gap between CSL and healthcare providers, managing inventory and delivery.
- Supply Chain Efficiency: Their operational expertise ensures timely and widespread access to CSL's therapies.
- Market Reach: Partnerships with distributors are key to CSL’s ability to serve a broad patient population.
- 2024 Market Value: The global pharmaceutical wholesale market's substantial size underscores the strategic importance of these relationships.
CSL's customer segments are diverse, ranging from patients with rare diseases requiring specialized biotherapies to healthcare providers who administer these treatments. Additionally, governments and public health organizations are key purchasers of vaccines for public health initiatives.
CSL Vifor specifically targets patients with iron deficiency, particularly those with chronic kidney disease, and also serves those with iron deficiency due to other chronic conditions like heart failure. Pharmaceutical distributors and wholesalers act as crucial intermediaries, ensuring CSL's products reach healthcare systems efficiently.
| Customer Segment | Key Characteristics | 2024 Relevance/Data Point |
|---|---|---|
| Patients with Rare Diseases | Require lifelong biotherapies for conditions like primary immune deficiencies and hemophilia. | CSL Behring's immunoglobulin therapies showed strong performance in niche markets. |
| Healthcare Providers | Physicians, specialists, and hospitals who prescribe and administer CSL's therapies. | Essential for patient access; demand for specialty medicines in immunology and hematology remains robust. |
| Governments & Public Health Orgs | Purchase vaccines for immunization programs and pandemic preparedness. | Continued investment in vaccine procurement and public health infrastructure in 2024. |
| Patients with Iron Deficiency | Especially those with CKD, on dialysis, or with heart failure. | Over 37 million adults in the US had CKD in 2024, many with iron deficiency. |
| Distributors & Wholesalers | Intermediaries managing logistics and product supply to healthcare facilities. | The global pharmaceutical wholesale market was valued around $1.5 trillion in 2024. |
Cost Structure
CSL dedicates a significant portion of its resources to Research and Development (R&D), covering everything from initial discovery to late-stage clinical trials for novel therapies and vaccines. This commitment involves substantial outlays for top scientific talent, advanced laboratory facilities, and strategic collaborations with external entities.
In fiscal year 2024, CSL's investment in R&D reached US$1.4 billion, underscoring its unwavering focus on driving innovation within the biopharmaceutical sector.
Operating CSL's extensive global network of plasma collection centers and managing the intricate processing of collected plasma constitutes a significant portion of its operational expenses. These costs encompass donor remuneration, the upkeep of facilities, essential equipment, and the employment of highly skilled personnel.
For instance, in fiscal year 2023, CSL's cost of goods sold, which includes these collection and processing expenses, was approximately $4.3 billion USD. The company is actively pursuing strategies, such as the implementation of the Rika automation system in its collection centers, to enhance operational efficiency and achieve cost reductions in this critical area.
CSL's manufacturing and production costs are significant, driven by the complex processes involved in creating biopharmaceutical products. These costs encompass essential raw materials, highly specialized and expensive equipment, substantial energy consumption for maintaining sterile environments, and the skilled labor required for operating regulated production facilities.
Maintaining stringent quality control and regulatory compliance across CSL's global manufacturing footprint represents a considerable ongoing expense. For example, CSL's Broadmeadows facility in Australia is a prime example of a major capital investment, underscoring the substantial upfront and operational costs associated with its advanced manufacturing capabilities.
Sales, Marketing, and Distribution Expenses
Reaching a global customer base for CSL, a leader in biotherapeutics, demands substantial investments in sales teams, diverse marketing campaigns, and critical medical education initiatives. These efforts are crucial for successful product launches and sustained market penetration.
The complexity of distributing life-saving therapies across varied geographies also adds significantly to this cost structure. CSL's commitment to patient access worldwide means navigating intricate supply chains and regulatory landscapes, driving up operational expenses.
For instance, in fiscal year 2023, CSL reported total selling, general, and administrative (SG&A) expenses of approximately $3.7 billion USD. This figure encompasses the broad range of activities required to bring their innovative treatments to patients globally.
- Global Sales Force: Maintaining a skilled international sales team to engage healthcare professionals and drive product adoption.
- Marketing and Advertising: Executing multi-channel campaigns to raise awareness and educate stakeholders about CSL's therapeutic areas.
- Medical Education: Funding programs to inform physicians and patients about the science and application of CSL's products.
- Distribution and Logistics: Managing complex cold-chain requirements and international shipping to ensure product integrity and timely delivery.
Regulatory Compliance and Quality Assurance
CSL faces significant expenses in maintaining compliance with stringent global health regulations and ensuring product quality. These costs are essential for market access and patient safety.
- Regulatory Filings and Approvals: Costs associated with preparing and submitting dossiers to agencies like the FDA and EMA, as well as ongoing fees for maintaining approvals. For instance, in fiscal year 2023, CSL's research and development spending, which includes significant regulatory components, was approximately AUD 1.5 billion.
- Quality Control and Assurance: Expenses for rigorous testing of raw materials, in-process samples, and finished products to meet pharmacopeial standards and internal quality benchmarks. This includes laboratory equipment, skilled personnel, and validation processes.
- Pharmacovigilance and Post-Market Surveillance: Costs for monitoring product safety after launch, collecting and analyzing adverse event reports, and implementing risk management plans. This ensures continued compliance and patient well-being.
CSL's cost structure is heavily influenced by its substantial investments in research and development, aiming to bring innovative therapies to market. Significant operational expenses are also incurred in managing its global plasma collection network and complex manufacturing processes. Furthermore, the company dedicates considerable resources to sales, marketing, and ensuring stringent regulatory compliance across its operations.
| Cost Category | Fiscal Year 2023 (Approx.) | Fiscal Year 2024 (Approx.) | Key Drivers |
|---|---|---|---|
| Research & Development (R&D) | AUD 1.5 billion (includes regulatory components) | US$1.4 billion | Talent, facilities, collaborations, clinical trials |
| Cost of Goods Sold (COGS) | US$4.3 billion | N/A | Plasma collection, processing, raw materials, manufacturing |
| Selling, General & Administrative (SG&A) | US$3.7 billion | N/A | Sales force, marketing, distribution, administration |
Revenue Streams
CSL Behring's global sales of plasma-derived therapies represent the company's largest revenue stream. This segment encompasses vital treatments like immunoglobulins and albumin, crucial for managing immune deficiencies and bleeding disorders. In fiscal year 2024, CSL reported strong performance in this core area, reflecting sustained demand for these life-saving therapies.
CSL Seqirus, a key player in global health, derives significant revenue from the worldwide sale of its seasonal and pandemic influenza vaccines. This includes innovative products such as FLUAD, a cell-based influenza vaccine offering enhanced protection, and FLUCELVAX, another cell-based option. The company's commitment to public health security translates into consistent demand for these vital immunizations.
Following its acquisition of Vifor Pharma, CSL Vifor generates significant revenue from its specialized portfolio of iron deficiency and nephrology products. This segment is crucial for CSL, offering treatments for conditions like chronic kidney disease and various iron-related disorders.
In fiscal year 2023, CSL reported that its combined revenue from the plasma and influenza businesses reached approximately $10.5 billion, with CSL Vifor's contribution being a key growth driver. The company is focused on unlocking long-term value and expanding its market presence within this therapeutic area.
Recombinant Product Sales
CSL's recombinant product sales are a significant revenue driver, particularly with innovative therapies like HEMGENIX, a gene therapy for hemophilia B. This segment showcases CSL's commitment to developing high-value, advanced treatments for rare diseases. While early adoption phases can influence immediate revenue figures, the long-term potential of these groundbreaking products is substantial.
The company's strategic investments in expanding its recombinant product pipeline are designed to capture future market growth. For instance, CSL Behring's focus on hemophilia treatments highlights a key area where recombinant technology offers a distinct advantage. These products are positioned as premium offerings, contributing to a diversified and high-margin revenue stream.
- Recombinant Product Portfolio: Includes advanced therapies for conditions like hemophilia B.
- Key Product Example: HEMGENIX, a gene therapy for hemophilia B, represents a significant innovation.
- Revenue Contribution: These high-value treatments contribute to the overall revenue mix, despite potential early uptake phases.
- Strategic Investment: CSL continues to invest in and expand its recombinant product area, signaling future growth potential.
Government Contracts and Pandemic Preparedness Agreements
CSL generates significant revenue by entering into direct contracts with governments and international bodies. These agreements are primarily focused on vaccine stockpiling and broader pandemic preparedness initiatives. This strategy provides CSL with a reliable and predictable income, underscoring its critical role in safeguarding global health security.
These strategic partnerships offer a consistent revenue foundation. For instance, CSL has established over 30 existing agreements with various governments specifically for influenza pandemic response. This demonstrates a proven track record and a substantial market presence in this vital sector.
- Government Contracts: Direct agreements with national governments for vaccine supply and preparedness.
- Pandemic Preparedness: Revenue from stockpiling and readiness programs for potential health crises.
- Stable Revenue: These contracts provide a predictable and recurring income stream.
- Global Health Security: Reflects CSL's position as a key player in international public health.
CSL Behring's plasma-derived therapies remain the cornerstone of its revenue. In fiscal year 2024, this segment continued to show robust performance, driven by consistent global demand for immunoglobulins and albumin used in treating immune deficiencies and bleeding disorders.
CSL Seqirus contributes substantially through its influenza vaccine sales, including innovative products like FLUAD and FLUCELVAX. The company's role in public health ensures ongoing demand for these critical immunizations, supporting its revenue generation.
The acquisition of Vifor Pharma has bolstered CSL's revenue streams with its specialized iron deficiency and nephrology products. This segment addresses significant unmet needs in chronic kidney disease and iron-related disorders, adding a vital therapeutic area to CSL's portfolio.
CSL's recombinant product portfolio, featuring advanced therapies like HEMGENIX for hemophilia B, represents a high-value, albeit newer, revenue stream. Strategic investments in this area are poised for long-term growth, showcasing CSL's commitment to cutting-edge treatments for rare diseases.
Direct government contracts for vaccine stockpiling and pandemic preparedness offer CSL a stable and predictable revenue base. With over 30 existing agreements for influenza pandemic response, this strategy highlights CSL's crucial role in global health security.
| Revenue Stream | Key Products/Services | Fiscal Year 2024 Commentary |
| Plasma-Derived Therapies | Immunoglobulins, Albumin | Strong performance, sustained demand for life-saving treatments. |
| Influenza Vaccines | FLUAD, FLUCELVAX | Consistent demand driven by public health needs and innovative offerings. |
| Iron Deficiency & Nephrology | Specialized treatments for iron disorders and kidney disease | Key growth driver following Vifor Pharma acquisition. |
| Recombinant Products | HEMGENIX (Hemophilia B gene therapy) | High-value, advanced treatments with significant long-term growth potential. |
| Government Contracts | Vaccine stockpiling, pandemic preparedness | Stable and predictable income from over 30 global agreements. |
Business Model Canvas Data Sources
The Business Model Canvas is meticulously crafted using a blend of financial statements, customer feedback, and competitive intelligence. These diverse data streams ensure a comprehensive and actionable strategic framework.