CareDx Porter's Five Forces Analysis
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
CareDx Bundle
CareDx navigates a complex landscape shaped by significant buyer power from transplant centers and intense rivalry within the diagnostics sector. The threat of substitutes, while currently moderate, could escalate as new technologies emerge.
This brief snapshot only scratches the surface. Unlock the full Porter's Five Forces Analysis to explore CareDx’s competitive dynamics, market pressures, and strategic advantages in detail.
Suppliers Bargaining Power
CareDx's reliance on specialized reagents and consumables for its molecular diagnostic tests positions suppliers with considerable bargaining power. These unique materials often originate from a restricted pool of manufacturers, making them difficult to substitute.
This scarcity can translate into significant leverage for suppliers, especially when switching costs for CareDx are substantial. For instance, in 2023, the cost of goods sold for CareDx was approximately $116.7 million, a figure directly influenced by the pricing of these critical inputs.
The dependency on these specialized components can directly impact CareDx's production expenses and the overall stability of its supply chain, potentially affecting its ability to meet market demand.
Suppliers of advanced molecular diagnostic equipment and proprietary technologies, such as next-generation sequencing (NGS) platforms, wield significant influence. CareDx's reliance on these specialized tools means suppliers can dictate terms, potentially impacting the company's cost structure and operational efficiency. For instance, the market for high-throughput NGS sequencers, crucial for transplant diagnostics, is dominated by a few key players.
CareDx's increasing adoption of AI-driven diagnostic tools, such as AlloSure Plus, heightens its dependence on specialized bioinformatics and AI software providers. These suppliers provide proprietary algorithms and data analysis capabilities essential for deciphering intricate genomic information.
The highly specialized nature of these software solutions often grants these vendors significant bargaining power. This can influence CareDx's ability to innovate and manage its operational costs, potentially impacting margins.
Highly Skilled Labor and Scientific Talent
The biotechnology and molecular diagnostics sector, where CareDx operates, is heavily reliant on a specialized workforce. This includes research scientists, geneticists, and skilled laboratory technicians, whose expertise is crucial for innovation and product development.
The limited availability of this highly skilled talent, often referred to as 'human capital,' can significantly enhance the bargaining power of these labor suppliers. This often translates into increased labor costs for companies like CareDx, impacting operational expenses.
Competition for top-tier talent is particularly intense, especially in cutting-edge areas such as the application of artificial intelligence in diagnostics. This heightened demand for specialized skills further amplifies the bargaining power of these skilled professionals, potentially driving up compensation and recruitment costs for CareDx.
- Talent Scarcity: The biotechnology industry faces a persistent shortage of specialized scientific and technical personnel.
- Wage Inflation: This scarcity directly contributes to upward pressure on wages and benefits for skilled labor, increasing operating costs for companies.
- AI Integration: The growing demand for AI expertise in diagnostics intensifies competition for a niche talent pool, further empowering these individuals.
Regulatory Compliance and Quality Control Materials
Suppliers of materials crucial for regulatory compliance and quality control hold considerable sway in the medical diagnostics sector. CareDx, operating in a heavily regulated environment, must adhere to strict standards, making it challenging to switch vendors if they consistently meet these demands. This reliance on compliant suppliers, while ensuring product integrity, can restrict sourcing flexibility.
For instance, in 2023, the U.S. Food and Drug Administration (FDA) continued to emphasize rigorous oversight of diagnostic test manufacturers. Companies like CareDx must invest in materials and services that guarantee adherence to these evolving regulations, such as specialized reagents and validated testing equipment. The cost associated with ensuring such compliance can be substantial, further empowering suppliers who offer certified and reliable components.
- Regulatory Hurdles: Navigating complex regulatory landscapes, such as those set by the FDA and EMA, necessitates specialized materials and services that few suppliers can provide.
- Quality Assurance: The need for consistent, high-quality inputs for diagnostic assays means CareDx is dependent on suppliers with proven track records in quality control.
- Limited Alternatives: Sourcing compliant materials often narrows the pool of potential suppliers, giving those who meet stringent requirements greater bargaining power.
Suppliers of specialized reagents and proprietary technologies, particularly for molecular diagnostics and AI-driven tools, hold significant bargaining power over CareDx. This is due to the limited availability of these critical inputs and the high switching costs involved. For example, CareDx's cost of goods sold in 2023 was approximately $116.7 million, directly reflecting the impact of input pricing.
The scarcity of specialized talent, such as AI experts and geneticists, further empowers labor suppliers, driving up recruitment and compensation costs for CareDx. The intense competition for these skills amplifies their leverage, impacting operational expenses.
Furthermore, suppliers of materials essential for regulatory compliance, like those meeting FDA standards, possess considerable influence. CareDx's need to adhere to strict quality controls and evolving regulations limits its sourcing flexibility, giving compliant vendors greater leverage.
| Input Type | Supplier Bargaining Power Factor | Impact on CareDx |
|---|---|---|
| Specialized Reagents | Limited availability, high switching costs | Increased cost of goods sold, supply chain stability |
| Proprietary AI/Bioinformatics Software | Unique algorithms, essential for diagnostics | Influence on innovation, operational costs, and margins |
| Skilled Scientific Talent | Talent scarcity, intense competition | Higher labor costs, increased recruitment expenses |
| Regulatory Compliant Materials | Strict quality and compliance requirements | Reduced sourcing flexibility, potential cost premiums |
What is included in the product
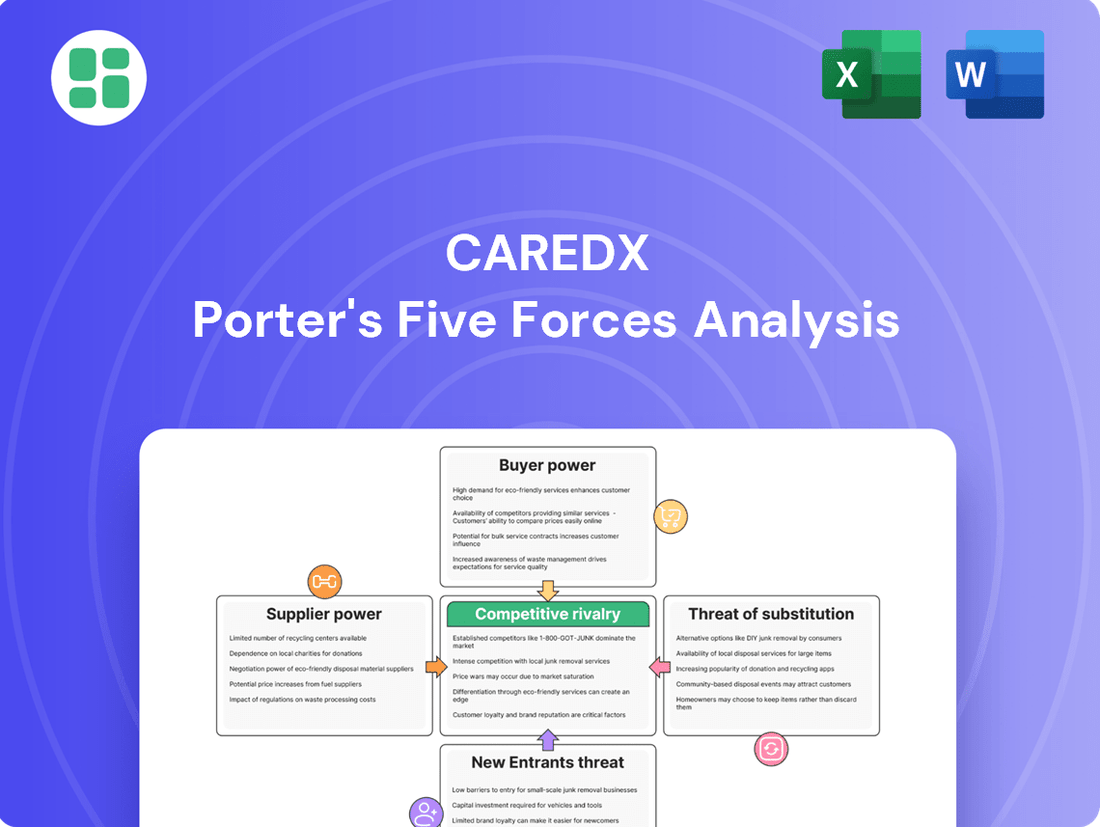
This Porter's Five Forces analysis offers a comprehensive examination of the competitive landscape for CareDx, detailing the intensity of rivalry, buyer and supplier power, threat of new entrants, and the impact of substitutes on the organ transplant market.
Instantly understand strategic pressure with a powerful spider/radar chart, simplifying complex competitive dynamics for CareDx.
Customers Bargaining Power
CareDx's primary customers, hospitals and transplant centers, are experiencing significant consolidation. This trend means fewer, but larger, entities are making purchasing decisions. For instance, by the end of 2023, the number of community hospitals in the U.S. had seen a continued decline, with larger health systems absorbing smaller ones, increasing their collective bargaining might.
These consolidated healthcare systems wield substantial purchasing power. Their ability to order a high volume of CareDx's diagnostic tests allows them to negotiate more aggressively on pricing and contract terms. This concentrated demand can create downward pressure on the prices CareDx can charge for its services.
Healthcare payers, such as Medicare and private insurers, hold significant sway over companies like CareDx through their reimbursement policies and prior authorization mandates. These entities dictate which diagnostic tests are covered and at what rate, directly affecting CareDx's revenue streams and ability to reach patients.
For instance, in 2024, Medicare's reimbursement rates for advanced molecular diagnostics, like those offered by CareDx, are a critical factor. A reduction in these rates, or stricter prior authorization criteria, could substantially limit CareDx's market access and profitability, underscoring the powerful bargaining position of these payers.
The bargaining power of customers for CareDx is influenced by their own internal capabilities. While CareDx specializes in molecular diagnostics for transplant patients, some larger transplant centers or reference labs possess the infrastructure for certain in-house testing. This potential for backward integration, even if partial, gives these customers leverage. For instance, if a large hospital system can perform a portion of the required tests internally, it reduces their absolute dependence on CareDx, potentially leading to price negotiations.
Focus on Patient Outcomes and Cost-Effectiveness
Customers in the healthcare industry, particularly patients and payers, are increasingly prioritizing tangible improvements in patient health alongside efficient cost management. This dual focus means they actively seek solutions that not only enhance treatment effectiveness but also contribute to overall healthcare affordability.
CareDx's products, such as its transplant diagnostics, are designed to offer crucial data that can lead to better long-term patient outcomes by monitoring organ health and predicting potential issues. This directly aligns with customer demands for solutions that improve quality of life and reduce the need for costly interventions.
Given this strong emphasis on value, customers are likely to exert significant bargaining power by demanding robust evidence of clinical utility and demonstrable cost-effectiveness. For instance, payers might require data showing a reduction in re-transplant rates or fewer hospital readmissions to justify the adoption of CareDx’s technologies. In 2023, the average cost of a kidney transplant in the US was approximately $466,000, highlighting the financial stakes involved in optimizing transplant success and longevity.
- Focus on Patient Outcomes: Healthcare providers and patients are driven by the need for improved graft survival rates and reduced complications post-transplant.
- Cost-Effectiveness Demands: Payers and health systems scrutinize the economic benefits, looking for data that supports a lower total cost of care over time, potentially through early detection of rejection or infection.
- Evidence-Based Value: Customers will leverage their purchasing power to insist on clinical trial data and real-world evidence demonstrating the superiority or cost-saving advantages of CareDx’s offerings compared to alternatives.
- Market Pressure: The drive for value places pressure on CareDx to continuously innovate and provide quantifiable benefits, making it harder to command premium pricing without clear proof of impact.
Switching Costs for Diagnostic Platforms
Customers switching from one diagnostic platform to another, like those using CareDx's offerings, might face some retraining or integration hurdles. However, the underlying infrastructure for molecular diagnostics is often shared, mitigating the full impact of these costs. For instance, a hospital lab already equipped with high-throughput sequencers might find the transition less burdensome than a complete overhaul.
Customers who have already invested in specific laboratory equipment compatible with CareDx's solutions or integrated its digital workflow tools will likely experience moderate switching costs. This investment can slightly temper their bargaining power in the immediate term, as the sunk costs make a quick pivot less appealing.
Despite these initial integration efforts, customers remain keenly aware of the long-term value proposition. If alternative platforms offer superior performance, cost-effectiveness, or broader diagnostic capabilities, the moderate switching costs will not prevent a shift if the perceived long-term benefits outweigh the costs of change.
- Moderate Switching Costs: Investments in specific lab equipment and digital workflow integration create barriers to switching.
- Shared Infrastructure: The commonality of molecular diagnostics infrastructure limits the magnitude of switching costs.
- Long-Term Value Sensitivity: Customers prioritize long-term benefits, potentially overriding moderate switching costs for superior alternatives.
CareDx faces significant customer bargaining power due to healthcare industry consolidation, leading to larger, more influential buyers. These consolidated entities can leverage their volume to negotiate better pricing, directly impacting CareDx's revenue. For example, the trend of hospital mergers continued through 2023, creating fewer but more powerful purchasing groups.
Payers, like Medicare and private insurers, also exert considerable influence through reimbursement policies and prior authorization requirements. In 2024, Medicare's reimbursement rates for advanced diagnostics are a key factor, with potential rate reductions or stricter approvals limiting CareDx's market access and profitability.
Customers are increasingly focused on both improved patient outcomes and cost-effectiveness. This dual demand means they will push for strong evidence of clinical utility and economic benefits. Given that the average cost of a kidney transplant in the US was approximately $466,000 in 2023, demonstrating value in improving long-term graft survival is crucial for CareDx.
| Customer Segment | Bargaining Power Drivers | Impact on CareDx |
|---|---|---|
| Hospitals & Transplant Centers | Industry Consolidation, High Volume Purchases | Downward pressure on pricing, demand for favorable contract terms |
| Healthcare Payers (Medicare, Insurers) | Reimbursement Policies, Prior Authorization, Coverage Decisions | Limits market access, affects revenue streams, requires demonstration of cost-effectiveness |
| Value-Conscious Customers | Demand for Improved Patient Outcomes, Cost-Effectiveness | Need for robust clinical utility data, pressure to prove long-term economic benefits |
Preview the Actual Deliverable
CareDx Porter's Five Forces Analysis
This preview showcases the exact CareDx Porter's Five Forces Analysis you will receive immediately after purchase, providing a comprehensive examination of industry competition and profitability. You're looking at the actual, professionally formatted document, ensuring you get a detailed breakdown of bargaining power of buyers, bargaining power of suppliers, threat of new entrants, threat of substitute products or services, and the intensity of rivalry among existing competitors.
Rivalry Among Competitors
The transplant diagnostics market is a battleground with both seasoned veterans and ambitious newcomers. CareDx finds itself in direct competition with a range of companies offering diverse diagnostic solutions, from other molecular diagnostics specialists to firms still relying on more traditional testing methodologies.
Established players like Thermo Fisher Scientific and Illumina, with their broad portfolios and extensive market reach, represent significant competitive forces. Emerging companies are also making their mark, often by focusing on niche areas or innovative technologies that challenge the status quo.
For instance, in 2024, the global transplant diagnostics market was valued at approximately $2.5 billion, with projections indicating steady growth. Companies like Eurofins Scientific and Quest Diagnostics also hold substantial market share, offering a wide array of testing services that can overlap with CareDx's offerings.
Developing and bringing advanced molecular diagnostics to market demands substantial upfront investment in research and development, alongside considerable fixed costs for laboratory setup and navigating regulatory pathways. For instance, companies in this space often spend millions on clinical trials and specialized equipment. This financial barrier can fuel intense competition as firms aim to capture market share and achieve economies of scale to justify these expenditures.
The transplant diagnostics market is seeing robust expansion, with projections indicating it could reach over $10 billion globally by 2028, fueled by rising transplant volumes and innovation. This upward trend in market size naturally draws in new entrants and spurs existing companies to ramp up their efforts to capture a larger slice of the pie.
This dynamic environment means that companies like CareDx face intense competition. For instance, the increasing demand for HLA typing and molecular diagnostics in transplantation creates opportunities for numerous players, from established biotech firms to specialized diagnostic companies, all vying for dominance.
Product Differentiation and Innovation
CareDx carves out its niche with specialized, genomics-based offerings like AlloSure and AlloMap, complemented by its AI-driven platforms. This focus on unique, high-value solutions sets it apart in the transplant diagnostics market.
However, the competitive landscape is dynamic, with rivals actively pursuing innovation. Competitors are introducing advanced technologies, including next-generation sequencing (NGS), liquid biopsies, and further AI integration, directly challenging CareDx’s established position.
The constant need to innovate and differentiate products is paramount for sustained competitive advantage in this sector. For instance, in 2023, the molecular diagnostics market, which includes CareDx's offerings, saw significant investment, with companies prioritizing R&D to stay ahead.
- CareDx’s core differentiators: AlloSure and AlloMap for transplant patient monitoring.
- Competitor innovation focus: NGS, liquid biopsies, and AI integration.
- Market trend: High R&D investment in molecular diagnostics to fuel innovation.
Regulatory and Reimbursement Landscape
The intricate and ever-changing regulatory and reimbursement environment for molecular diagnostics significantly shapes competitive rivalry. Companies must successfully navigate rigorous approval pathways and secure favorable reimbursement terms, creating substantial barriers for smaller entrants. This landscape also becomes a critical battleground for market access among established, larger competitors.
For instance, in 2024, the Centers for Medicare & Medicaid Services (CMS) continued to refine its coverage policies for advanced diagnostics, impacting market adoption. Companies like CareDx, which focus on transplant diagnostics, must continuously adapt to these evolving reimbursement codes and coverage decisions, directly influencing their revenue streams and competitive positioning.
- Navigating FDA Approvals: Stringent FDA review processes for new diagnostic tests, particularly those involving novel biomarkers or complex methodologies, act as a significant hurdle. Meeting these requirements demands substantial investment in clinical validation and regulatory affairs expertise.
- Reimbursement Dynamics: Securing adequate reimbursement from payers like Medicare and private insurers is crucial. In 2024, many molecular diagnostic tests faced scrutiny regarding their clinical utility and cost-effectiveness, leading to varied coverage decisions that can create uneven playing fields.
- Market Access as a Differentiator: Companies that can effectively demonstrate the clinical utility and economic value of their diagnostic solutions are better positioned to gain broad market access and favorable reimbursement rates, thereby strengthening their competitive advantage.
Competitive rivalry within the transplant diagnostics sector is intense, driven by significant market growth and technological advancements. Companies like CareDx face direct competition from large, established players such as Thermo Fisher Scientific and Illumina, as well as emerging firms pushing innovative solutions. The global transplant diagnostics market, valued around $2.5 billion in 2024, is projected for substantial expansion, attracting further competition.
| Competitor | Key Offerings | 2024 Market Presence/Focus |
|---|---|---|
| Thermo Fisher Scientific | Broad diagnostic portfolio, HLA typing | Significant market share, extensive global reach |
| Illumina | NGS-based solutions, genetic analysis | Leading in sequencing technology, expanding into diagnostics |
| Eurofins Scientific | Comprehensive testing services, molecular diagnostics | Strong presence in clinical diagnostics, broad service offering |
| Quest Diagnostics | Extensive laboratory network, various diagnostic tests | Major player in clinical pathology, growing molecular capabilities |
| CareDx | AlloSure, AlloMap (transplant monitoring), AI platforms | Specialized focus on transplant patient care, innovation-driven |
SSubstitutes Threaten
Traditional organ biopsies, while invasive, are a long-standing benchmark for monitoring transplanted organs. Despite the advancements in non-invasive molecular diagnostics like those offered by CareDx, the established clinical reliance on biopsy as the perceived gold standard presents a significant substitute threat. Clinicians often weigh the invasiveness of biopsies against the diagnostic certainty they provide.
Beyond CareDx's proprietary dd-cfDNA technology, the threat of substitutes includes other biomarker-based diagnostic methods. These could involve assays detecting different molecular markers or protein signatures that indicate organ health or rejection. For instance, research into circulating tumor DNA (ctDNA) for cancer monitoring shares technological similarities and could potentially be adapted for transplant applications, offering an alternative approach to detecting cellular damage or immune response.
The emergence of novel biomarker panels, such as those focusing on specific inflammatory cytokines or immune cell populations, presents another substitute threat. If these alternative tests demonstrate comparable or superior accuracy in predicting organ health and rejection events, particularly with improved cost-effectiveness, they could erode CareDx's market share. For example, advancements in proteomics could yield protein-based biomarkers that are easier to detect or more directly indicative of specific cellular processes, challenging the dominance of DNA-based methods.
The growing field of non-invasive diagnostics presents a potential threat to CareDx. Innovations like advanced imaging, organ-on-a-chip technologies, and new biosensors are emerging. While not immediate replacements, these advancements could offer alternative ways to monitor transplant health over time, potentially lessening the need for current molecular diagnostic solutions.
Clinical Assessment and Patient Symptoms
In certain scenarios, particularly in resource-limited environments or for initial patient triage, a clinical assessment relying on observable symptoms, physical examinations, and fundamental laboratory tests can function as a low-cost substitute for sophisticated diagnostic tools. This less precise method might lead to a delay or even a complete avoidance of more expensive molecular testing, thereby influencing the market penetration of advanced diagnostics.
Consider the impact on diagnostic test adoption: a basic clinical evaluation, while lacking the specificity of advanced molecular diagnostics, can serve as an initial screening mechanism. This might reduce the volume of patients referred for more costly tests, especially if healthcare providers are cost-sensitive.
The threat of substitutes in this context can be quantified by observing trends in healthcare spending versus diagnostic test utilization. For instance, if overall healthcare expenditure growth outpaces the adoption of advanced diagnostics, it could indicate a greater reliance on less sophisticated, lower-cost assessment methods.
- Basic clinical assessment as a low-cost alternative to advanced diagnostics.
- Potential for delayed or reduced demand for molecular tests due to symptom-based evaluation.
- Impact on market adoption of advanced diagnostic solutions in resource-constrained settings.
Pharmacogenomics and Personalized Immunosuppression
Advances in pharmacogenomics are creating more precise personalized immunosuppression regimens. This precision could reduce rejection rates, potentially lessening the need for intensive, frequent monitoring. For instance, tailored drug therapies might decrease the demand for extensive post-transplant diagnostic testing, acting as an indirect substitute for current diagnostic intensity.
The development of highly effective, individualized immunosuppressant drugs poses a threat. If these therapies significantly improve patient outcomes and reduce complications, the reliance on broad diagnostic monitoring services could diminish. This shift could impact companies like CareDx, which offer specialized diagnostic solutions for transplant patients.
- Pharmacogenomic advances: Tailored drug therapies reduce the need for broad diagnostic monitoring.
- Reduced rejection rates: Personalized regimens may lower the incidence of organ rejection.
- Indirect substitution: Effective drug treatments substitute for intensive diagnostic testing.
- Market impact: Companies focused on diagnostic intensity may face reduced demand.
While molecular diagnostics are advancing, traditional organ biopsies remain a significant substitute. Their long-standing use as a benchmark means clinicians are accustomed to their diagnostic certainty, even with their invasiveness. This established practice presents a hurdle for newer, non-invasive methods.
Other biomarker-based diagnostics, beyond CareDx's dd-cfDNA, also pose a threat. These could involve different molecular markers or protein signatures that indicate organ health. For example, advances in proteomics might yield protein-based biomarkers that are easier to detect, potentially challenging DNA-based methods.
The threat of substitutes also includes less sophisticated, lower-cost assessment methods. Basic clinical evaluations and symptom-based assessments, especially in resource-limited settings, can delay or reduce the demand for advanced molecular tests. This impacts market adoption for companies like CareDx.
Furthermore, advancements in pharmacogenomics, leading to more precise immunosuppression, could indirectly substitute for intensive diagnostic monitoring by reducing rejection rates. For instance, if tailored drug therapies significantly improve outcomes, the reliance on broad diagnostic services might decrease.
Entrants Threaten
Entering the molecular diagnostics market, particularly for specialized areas like transplant monitoring, demands significant capital outlay. This includes substantial investment in research and development to create innovative solutions, rigorous clinical trials to prove their efficacy, and the establishment of advanced laboratory infrastructure. For instance, the development of a new diagnostic test can easily cost tens of millions of dollars, with ongoing R&D for next-generation products adding to this burden.
These high upfront costs act as a formidable barrier, deterring many potential new players from entering the field. Companies must secure considerable funding to navigate the complex regulatory landscape and bring a new diagnostic product to market. Without this financial muscle, aspiring entrants find it incredibly challenging to compete with established firms that have already made these investments.
The medical diagnostics sector, including companies like CareDx, faces substantial barriers to entry due to stringent regulatory requirements. Obtaining approvals, such as FDA clearance in the United States, necessitates extensive clinical validation and adherence to complex guidelines. This process demands significant investment in time, capital, and specialized expertise, making it challenging for new players to emerge and compete effectively.
Established players like CareDx leverage a robust intellectual property and patent portfolio, encompassing key technologies such as AlloSure and AlloMap. This extensive patent protection acts as a significant hurdle for newcomers, compelling them to either innovate entirely new solutions or seek costly licenses, thereby increasing their entry barriers and legal exposure.
Brand Recognition and Established Clinical Relationships
CareDx has cultivated deep-seated relationships with transplant centers, clinicians, and patients, leading to significant brand recognition and trust within the specialized transplant ecosystem. This established network makes it difficult for new companies to penetrate the market.
New entrants would need to overcome substantial hurdles to gain credibility and build necessary distribution channels. The conservative nature of the medical community, which prioritizes proven clinical utility and extensive long-term data, presents a formidable barrier to entry for any new player in this field.
- Established Trust: CareDx's long-standing presence has fostered deep trust among key stakeholders in the transplant community.
- Clinical Relationships: The company has built strong, enduring ties with transplant centers and healthcare professionals.
- Barriers to Credibility: New entrants face a significant challenge in establishing credibility within a field that values proven, long-term clinical data.
- Distribution Hurdles: Gaining access to established distribution networks and acceptance by a conservative medical community is a major obstacle for newcomers.
Access to Specialized Data and Bioinformatics Expertise
Developing cutting-edge molecular diagnostics, particularly those leveraging artificial intelligence, hinges on access to extensive, specialized clinical data and profound bioinformatics acumen for analysis and algorithm creation. Newcomers face considerable hurdles in acquiring or generating proprietary datasets and attracting the requisite talent, creating a substantial barrier to entry.
For instance, the development of advanced AI algorithms for transplant rejection prediction, a core area for companies like CareDx, demands years of meticulously curated patient data and specialized machine learning expertise. In 2024, the demand for skilled bioinformaticians and data scientists in the life sciences sector remained exceptionally high, with reported salary increases of 15-20% for experienced professionals in this niche, making talent acquisition a significant challenge for any new player.
- Data Acquisition Costs: Securing access to large, high-quality clinical datasets can involve substantial licensing fees or the significant investment required to build internal data generation capabilities.
- Talent Scarcity: The pool of individuals with both deep bioinformatics knowledge and practical experience in developing diagnostic algorithms is limited, driving up recruitment costs and timelines.
- Regulatory Hurdles: Navigating the complex regulatory landscape for novel diagnostic tools, especially AI-driven ones, requires specialized expertise and significant time, further deterring new entrants.
The threat of new entrants for CareDx is considerably low due to immense capital requirements for R&D, clinical trials, and infrastructure, often exceeding tens of millions of dollars for a single diagnostic test. Stringent regulatory hurdles, like FDA approvals, add significant time and cost, making it difficult for newcomers to navigate the complex landscape. Furthermore, established intellectual property and patent portfolios protect existing technologies, forcing new players to either innovate extensively or incur licensing fees, thus increasing entry barriers.
New entrants face significant challenges in building credibility and distribution channels within the transplant ecosystem. The medical community's preference for proven, long-term clinical data and the conservative nature of adoption create a substantial obstacle. CareDx's established relationships with transplant centers and clinicians foster deep trust, making it hard for new companies to gain traction and market access.
Access to specialized clinical data and skilled bioinformatics talent is a major barrier, particularly for AI-driven diagnostics. Acquiring or generating proprietary datasets and attracting experts in data science and machine learning is costly and time-consuming. In 2024, the demand for experienced bioinformaticians saw salary increases of 15-20%, highlighting the talent scarcity and recruitment challenges for new entrants.
| Barrier Type | Description | Impact on New Entrants |
|---|---|---|
| Capital Requirements | High costs for R&D, clinical trials, and lab infrastructure. | Deters companies lacking substantial funding. |
| Regulatory Hurdles | Complex approval processes (e.g., FDA). | Increases time, cost, and need for specialized expertise. |
| Intellectual Property | Existing patents on key technologies. | Forces licensing or costly alternative innovation. |
| Established Relationships | Strong ties with transplant centers and clinicians. | Makes market penetration and trust-building difficult. |
| Data & Talent Access | Need for specialized clinical data and bioinformatics expertise. | High acquisition costs and talent scarcity create significant challenges. |
Porter's Five Forces Analysis Data Sources
Our CareDx Porter's Five Forces analysis is built upon a foundation of comprehensive data, including SEC filings, investor presentations, and industry-specific market research reports to accurately assess competitive dynamics.